

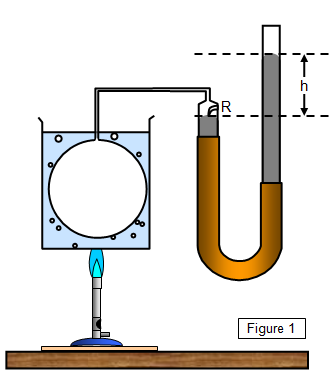
A simple
form of constant-volume gas thermometer is shown in Figure 1. The gas is enclosed in the
bulb B and the pressure recorded by the difference in levels (h) of the mercury columns. The
mercury level at R is always adjusted so that it coincides with the mark. The pressure of the
gas within the bulb is then given by P = A + h, where A is the atmospheric pressure.
If the atmospheric pressure varies during the experiment allowance must be
made for this, since it is the total gas pressure that is
measured.
The gas in the bulb can be air, hydrogen, helium or nitrogen,
although it is the constant-volume hydrogen gas thermometer that is taken as
standard.
The simple form of constant-volume gas thermometer is subject to errors
due to changes in volume of the glass and of the mercury (due to temperature variations), to
pressure on the bulb and to the exposed column 'dead space', that is, the volume of gas that
is outside the region of which the temperature is being measured. It has the further
disadvantages that it is not direct-reading, and that it cannot be used to measure varying
temperatures, because gases are such poor conductors of heat.
A more accurate
form of constant-volume thermometer has been designed where some of these errors are
reduced, the dead space is made as small as possible and the bulb containing the gas is
large (1.6 litres).
By using different gas thermometers a wide range of temperatures
can be measured:
Hydrogen -200 oC to +500
oC
Nitrogen +500 oC to + 1500 oC
Helium
-270 oC to + 1500 oC
These thermometers can be
very accurate, to within 0.005 oC from 0 oC to 100 oC, 0.1 oC
around 500 oC and to within 2 oC at 1500 oC